Medical milestone: Thailand unveils first domestic targeted cancer drugs
Medical milestone: Thailand unveils first domestic targeted cancer drugs
วันที่นำเข้าข้อมูล 8 ส.ค. 2568
วันที่ปรับปรุงข้อมูล 8 ส.ค. 2568
Thailand has reached a historic milestone in public health with the domestic development of its first targeted oral cancer drugs, dramatically increasing treatment accessibility and reducing reliance on costly imports.
Central to this achievement is the vision of Her Royal Highness Princess Chulabhorn Krom Phra Srisavangavadhana, whose decades-long dedication to medicine and compassionate leadership has led to the launch of two advanced cancer medications: IMCRANIB 100 and HERDARA.

IMCRANIB 100, approved by Thailand’s Food and Drug Administration (FDA) in May 2025, represents a new frontier in Thai pharmaceutical innovation. Developed without foreign technology transfers, it inhibits the tyrosine kinase enzyme that drives cancer growth, offering precise and less toxic treatment for several serious conditions, including:
- chronic myeloid leukemia
- Philadelphia chromosome-positive acute lymphoblastic leukemia
- gastrointestinal stromal tumors
- rare skin cancers like DFSP
While a similar imported drug, imatinib, is partially reimbursed under national health schemes, its high cost and limited coverage have made it inaccessible for many. IMCRANIB 100 aims to fill that gap, offering a Thai-produced, cost-effective alternative that broadens treatment eligibility and improves quality of life for cancer patients nationwide.
The Chulabhorn Model

This pharmaceutical leap is the direct result of Her Royal Highness’s 2020 establishment of the Royal Pharmaceutical Manufacturing Plant at Phimanmas Residence in Chonburi Province. Certified to international GMDP PIC/S standards, the facility is the first of its kind in Thailand — a fully integrated cancer drug plant capable of taking research from lab benches to bedsides.
IMCRANIB 100 is already being adopted by Chulabhorn Hospital, Thailand’s leading oncology center, which began administering the drug in July 2025. With robust systems for clinical monitoring, medication management, and patient support, the hospital serves as a vital bridge between science, production, and real-world care. Physicians, pharmacists, and researchers are working together to track outcomes and ensure safety in what is essentially a national pilot program.
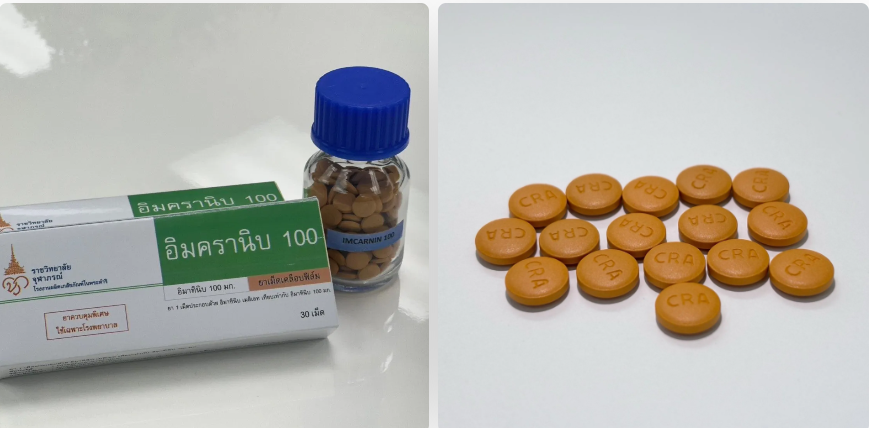
Alongside IMCRANIB 100, the development of HERDARA — a domestically produced version of the breast cancer drug trastuzumab — is also reshaping access. Previously costing up to THB1 million (approx. US$30,800) per course, HERDARA dramatically lowers treatment costs for patients relying on Thailand’s universal healthcare system.
More than scientific milestones, these developments reflect a strategic transformation in Thailand’s approach to health equity, sustainability, and pharmaceutical independence, including via vertical integration. Under Princess Prof Dr Chulabhorn’s continued patronage, Thailand is not only saving lives, but setting a blueprint for emerging economies seeking medical self-sufficiency in an age of soaring drug prices.
Thailand Business Information Center in Taiwan